The young woman removes a round container from the packaging and places it carefully on her child’s upper arm. With her thumb, she releases a boost that launches a tiny patch onto the surface of the skin. The patch is equipped with thousands of needles that are coated with the necessary vaccine.
According to information from the World Health Organization (WHO), as well as other NGOs, non-profits and private groups, conventional vaccination campaigns will be supplemented with such microneedle array patches (MAPs) in the future. They herald the end of syringes that can only be applied by qualified specialists. The end of liquid vaccines that require a continuous cooling chain. Especially in low and middle income countries, simple vaccination using microneedle patch is a promising alternative. The Australian start-up VAXXAS and Harro Höfliger have been working to implement this technology, with a view to the cost aspect as well as the upscaling process.
Many paths lead to the summit
Microneedles are attractive for a number of markets: vaccination campaigns in developing countries are one area, but another is for more upmarket products such as migraine remedies. A diverse array of microneedle technology development teams and researchers are following different strategies in terms of choice of material as well as the application and delivery of the active substance.
Coated microneedles
The microneedles made of metal or plastic are coated with a liquid active substance that dissolves later in the skin. To this end, they are either immersed in an active ingredient solution or imprinted and then dried.

Self-absorbing microneedles
The microneedles are formed from a suitable polymer (PVP, PVOH) and the active ingredient to be administered. When manufactured with a pressure process, the needles are already a component of the support patch, while in other manufacturing processes they are glued on in a later step. The needles dissolve completely once they penetrate the skin, giving off their active ingredient.
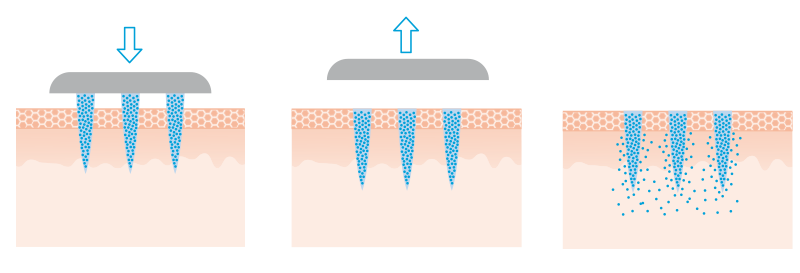
Hollow microneedles
Hollow microneedles (HM) are intended for the administration of higher doses of active substances. Channels are drilled into the stainless steel needles, for example with a laser. The active ingredient solution stored in a reservoir on the carrier film passes through the hollow needles into the skin.

An eye for the possible
The technology company, which came into being as an offshoot of the Australian Institute of Bioengineering & Nanotechnology of the University of Queensland, concentrates on novel vaccine delivery technologies. The Nanopatch™ that VAXXAS has developed is mainly designed for well-known vaccines.
Right from the early stages, the interdisciplinary research team wanted to make sure that the ideas and processes from their laboratory studies would be reliably translated into high-volume series production. Mike Junger, Head of Medical Device and Process Engineering at VAXXAS, explains: “That’s why we got in touch with Harro Höfliger, and we are really pleased that the experts were quickly ready to assist us in development of the device with a view to subsequent upscaling of the production process.”
For Stefan Bernsau, Director of Needle Technology at Harro Höfliger, the collaboration has been a clear win-win solution:A big part of our philosophy as a company is guiding our customers from the laboratory into production. That is why we often lend our expertise and resources to start-ups.” The project with VAXXAS links several of Harro Höfliger’s technology platforms: aseptic assembly, automation, and filling and dosing technology.
Bernsau: “It is a highly demanding process development, since between the coating and sealing you have to include the drying process of the different active substances under aseptic conditions.”
“It is a great technology which is going to simplify vaccination.“ Stefan Bernsau, Director Needle Technology at Harro Höfliger
Harro Höfliger has been engaged in the topic of microneedle patches for quite some time and Stefan Bernsau regularly participates in conferences on this topic, including those of the WHO. He has built up a worldwide network that is working together to find practical solutions for this technology of the future.
Bernsau: “It is a great technology with many challenges and it is going to simplify vaccination considerably. The consistent approach to new processes and developments together with VAXXAS, taking into account aseptic requirements, corresponds exactly to Harro Höfliger’s philosophy.”
Targeted protection
Recent studies have shown that intradermal vaccination may have advantages over the previously customary administration of vaccines into the subcutis or muscle. In contrast to the subcutis and the muscle, where only a few defense cells of the immune system are located, defense cells of the dendritic type are very common in the upper skin layer (dermis). These cells are responsible for initiating the immune defense.
If the vaccine active ingredient is administered here, it ensures a stronger immune response and thus improved vaccine protection. Microneedle patches also follow this approach. VAXXAS aims to achieve a more targeted administration of lower vaccine doses in order to optimize their effectiveness.

Going skin deep
The Nanopatch™ from VAXXAS consists of a one-centimeter polymer square that contains several thousand micro projections, each just 0.25 millimeters high. These are coated with the vaccine, which they inject directly into the subcutaneous layers that are rich in immune cells. Thanks to the applicator, the Nanopatch™ can be applied quite easily, even by non-specialists. The special design ensures that the vaccine is consistently administered, regardless of age- and sex-related differences in skin structure. Cooling the active substance is not necessary, thanks to its solid physical form.
“In future, nanotechnology will be the norm in sterile manufacturing.“ Mike Junger, Head of Medical Device and Process Engineering bei VAXXAS
“We have a ‘do it yourself’ corporate philosophy,” says Mike Junger. “I trust my employees’ abilities. They understand our product and they have the tools to do their job better than anyone else. We therefore didn’t expect Harro Höfliger to solve all the problems on their own. It was important for us to develop our ideas further by collaborating with the machine building specialists with all of their experience, which guaranteed practical implementation in the end.”
The hard facts

When developing an upscalable
process for future series production of the device, VAXXAS relies on the know-how of Harro Höfliger’s mechanical engineering specialists.
VAXXAS has now had their vaccine coating process for the microneedles patented. The next challenging task is to build a system on which the VAXXAS technology can be automatically and cost-effectively realized in large-scale production. The largest challenges are the highly precise coating of the needles with vaccine, the subsequent drying process and the sealing, all of which have to be carried out under aseptic conditions. According to Bernsau, “in order to apply a large enough amount of active substance, the needles need to be coated multiple times. To do this, we need a non-contact, highly precise, automated and camera-monitored dosing system that works in a sterile environment.”
This work is in progress. The VAXXAS brain trust and the specialists at Harro Höfliger are in constant contact to resolve issues as they arise. Junger explains: “We believe that in the future, micro- and nanotechnology will be the norm in sterile manufacture. But we still need to convince the regulatory authorities and the industry so that guidelines and standards can be adapted. Our Nanopatch™ is an important contribution to the process.”
About VAXXAS
![]() VAXXAS is a technology start-up founded in 2011 based on research at the Australian Institute of Bioengineering & Nanotechnology at the University of Queensland and is engaged in improving the performance of vaccines by delivery into the skin by the Nanopatch.
VAXXAS is a technology start-up founded in 2011 based on research at the Australian Institute of Bioengineering & Nanotechnology at the University of Queensland and is engaged in improving the performance of vaccines by delivery into the skin by the Nanopatch.
Download this article as PDF file
Photos: shutterstock.com/Cheers Group, Janine Kyofsky, Harro Höfliger
