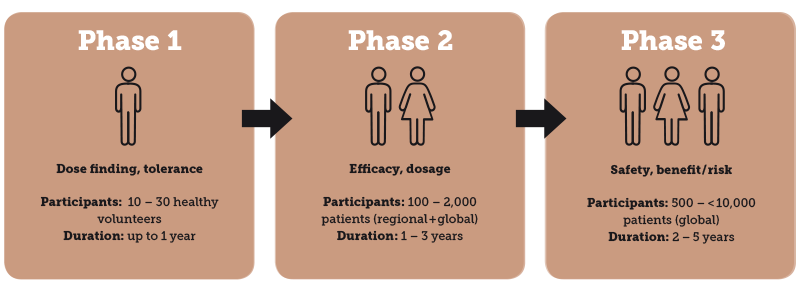
The production of clinical trial materials can present a real challenge, especially for small pharmaceutical companies, niche providers and start-ups: Small batch sizes must be produced under strict GMP guidelines (depending on the trial phase, between 10 and 10,000+ patients participate in the studies).
Having the right equipment technology is another key component when it comes to successfully producing clinical trial supplies. However, investment in the appropriate equipment at the clinical trial phase can be cost prohibitive for many small pharmaceutical companies. A potential solution is to find a contract service provider who can offer the right equipment technology, understands the importance of a scalable process, and can cater to the needs of small and specialty pharmaceutical clients.
“The Harro Höfliger principle is based on comprehensive manufacturing processes from the laboratory to production scale.“ Dr. Justin Lacombe, Director Pharmaceutical Development at Experic
Unfortunately, given the number of mergers and acquisitions in this field over the past few years there are fewer alternatives to choose from and it has become more challenging for this segment of pharmaceutical companies to find the right partner.Experic, a US based company started to address this problem in early 2019. As a partner for smaller companies, Experic manufactures and packages clinical trial materials and specialty commercial supplies on the customer’s behalf. Equipped with state-of-the-art dosing machines from Harro Höfliger and a blister machine from Uhlmann, Experic covers the entire spectrum of capsule and blister filling.
“The Harro Höfliger principle is based on comprehensive manufacturing processes from the laboratory to production scale,” explains Dr. Justin Lacombe, Director Pharmaceutical Development at Experic. “Following positive clinical results using drug products made on pilot-scale equipment, product and manufacturing process know-how can be seamlessly transferred to Harro Höfliger production systems with the same underlying technologies. The combination of a consistent single source and critical technology reduces the risk of having to modify the process at a later stage, saving time and, of course, money.”
The clinical trial phases
Plans for the formation of Experic date back to 2015. During this early phase, Harro Höfliger was already involved in the project and later became an investor. “With Experic, we see a huge potential for our US customers,” says Peter Brun, Team Leader Pharma Services at Harro Höfliger. “The facility closes an important gap in the production and the primary and secondary packaging of small series of pharmaceuticals in the US. Moreover, it serves as a high-tech showroom where customers can experience our technology live.”
The comprehensive service offering not only includes production, but also a GMP warehouse, DEA vault and worldwide distribution of clinical trial materials. “With Experic, start-ups and smaller pharmaceutical enterprises gain an experienced partner who can guide them with professional expertise during clinical studies all the way to product approval,“ explains Thomas Weller, CEO at Harro Höfliger.
Download this article as PDF file
Photo: Experic