Since the successful use of mRNA vaccines against COVID-19, awareness of biotechnological medications has spread beyond the circle of experts. The pharmaceutical industry has been increasingly using highly effective and patient specific therapeutic approaches for years in the fight against diseases.
Since 2012, EMA (European Medicines Agency) and the US FDA (Food and Drug Administration) have approved several gene therapeutics that can be used to adjust genetic defects in order to cure diseases. In the next three years the FDA expects around 800 new licenses. The sensitive products, often even living cells, are generally administered intravenously, and are extremely challenging to handle. They not only require aseptic production but also sterile filling in bags, syringes or vials.
“Our motto ‘the product determines the process’ is right on the nail here. We devote all of our expertise to the development of aseptic production processes.” Christian Kollecker, Director Aseptic Technologies at Harro Höfliger
Harro Höfliger has been dealing for years with the challenges of the bridge between flexible primary packaging and the demanding production requirements for aseptic bag filling. Christian Kollecker, Sales Director Aseptic Technologies at Harro Höfliger, explains: “Our motto ‘the product determines the process’ is right on the nail here. We draw on our full know-how for developing aseptic production processes. Together with our partners we develop completely new machine platforms to offer our customers unique and individually configurable solutions.”
New territory? Only partly!
In designing aseptic equipment, Harro Höfliger’s process and machine developers can draw on their know-how and experience with many issues.
Julian Grossman, Sales Manager Aseptic Technologies at Harro Höfliger, explains: “We’re already exclusively building specialty machinery. Every machine differs from project to project, even if only minimally at times. This is even more evident in the aseptic sector. It’s almost impossible to establish standards. In this sector our established approach of collaborating closely with customers to develop a holistic solution individually designed for their needs is unavoidable.”
Fundamentally, the processes in developing an aseptic machine are no different from those already established at Harro Höfliger. Additionally, it is essential in this product sector to ensure that the sterile product – here the active ingredient and, for example, the bag it is filled in – remain sterile throughout the entire filling process.
BU
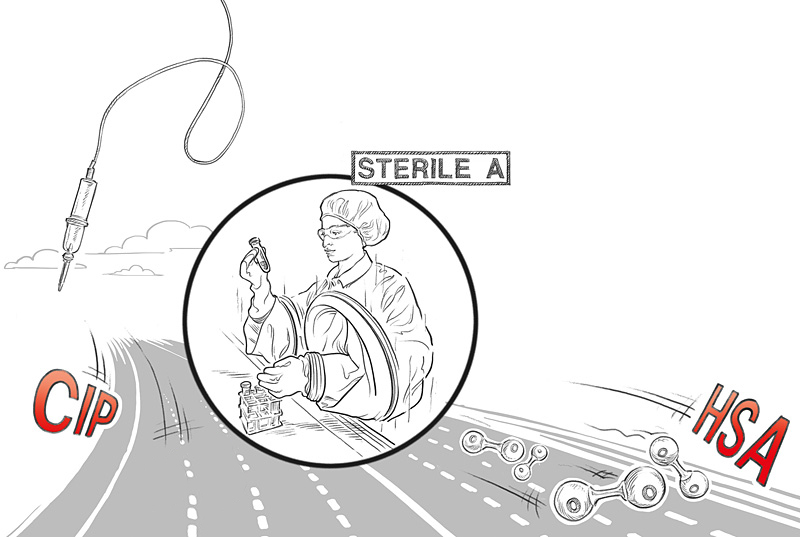
Christian Kollecker describes just some examples that developers have to bear in mind: “It must be possible to bring in all presterilized components from outside, i.e. the ‘dirty’ area, so that they remain sterile within the machine. It is also necessary to determine which cleaning and decontamination procedures are required to guarantee sterility throughout the entire process. The decontamination media also require modification of the machine design. They are mostly liquid or gaseous and would lead to corrosion at edges open downwards after a single cleaning cycle. Naturally, the machine operator may only reach into the machine through special glove systems.”
A resounding success through know-how
BU
Harro Höfliger proved that these requirements can be met as early as 2018, when it developed an aseptic filling and sealing line for presterilized IV bags for a biotech company in Hong Kong which is specialized in products for veterinary medicine. Completely sterile processes were needed for filling the highly sensitive bioactive ingredients.
Besides an active open RABS technology (Restricted Access Barrier System), this machine also used the ‘one-time docking’ principle for the first time. In this the IV bags are filled using an aseptic triple filling head. The connection to the filling nozzle is kept in place throughout all gas purging, evacuation and filling processes until the transfer from the machine. This minimizes the risk of particle entry and keeps the residual oxygen content of the bag low.
An integrated, laser-based Wilco HSA (Head Space Analysis) module provides an in-line measurement of the oxygen content. A high-precision measurement of mass flow using the Coriolis effect is performed during liquid filling by a sensor which ensures that each infusion bag has been filled to exactly the right amount.
BU
All fill media are supplied in a sterile manner by aseptic rotary distribution. The opening is tightly sealed by radio frequency welding to minimize the heat transfer to the active ingredient. An integrated CIP/SIP system (Cleaning in Place/Sterilization in Place) ensures perfect cleaning after each batch.
Christian Kollecker summarizes: “In this project we implemented a lot of things which stricter regulations would soon make standard practice for several of our customers. And this showed us what the important things were for aseptic machines: Short throughput times and high process reliability by integrating process analytical technology wherever possible and necessary.”
Safely filled
BU
The specialists at Harro Höfliger focus on filling presterilized bags. Kollecker explains: “Naturally, we can also fill syringes and vials, but the large, long-chain molecules of biotech products often form highly viscous solutions that have to be diluted to larger volumes. Infusion bags have clear advantages here.”
The experts especially have a wealth of experience with filling presterilized bags. As Grossmann knows, the devil here is again in the details: “Dealing with the new substances is new territory for us as well. Whether it is living cells or so-called lyophilized powders that emerge like cotton candy, we have to develop the right filling method for each substance.”
To deliver good solutions to customers quickly, Harro Höfliger relies on flexibility through modularity. Kollecker explains: “We develop prefabricated modules which can be quickly integrated into flexibly configurable machine platforms. This moves away from classic mechanical engineering and brings us closer to our customers as system providers.”
“We, too, are entering unchartered territory when it comes to dealing with these new substances. Be it living cells or lyophilized powders – we have to develop the right filling method for each substance.” Julian Grossmann, Sales Manager Aseptic Technologies at Harro Höfliger
Whether in bags, syringes or vials – antiseptically produced and filled active ingredients are the future. And Harro Höfliger is well placed for this. Christian Kollecker is confident: “We have a good overview and are able to visualize a customer process fully with Harro Höfliger technology. What our customers get from us in the aseptic sector is a fully-integrated system for their specific requirements – from a laboratory machine to a commercial production line.”
Download this article as PDF file
Photos: Janine Kyofsky, Illustration: Bernd Schifferdecker