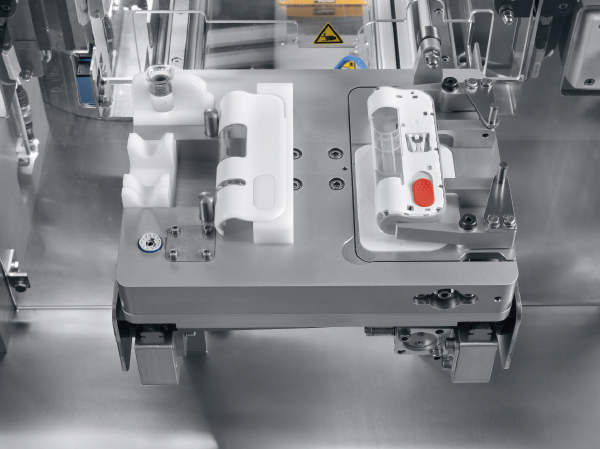
When manufacturing medical and pharmaceutical devices, nothing is more critical than process stability. That is why Harro Höfliger starts with defining a suitable production process for their customers and provides product-oriented solutions for the clinical trial stage and small series production – such as for the Libertas™ by Becton Dickinson (BD). This portable injector adheres to the skin and enables patients to self-inject subcutaneously.

BD Libertas™ incorporates BD Neopak™ primary container technology and employs the same cannula technology found in BD’s needles. The design and interface of the wearable injector are the result of extensive preclinical and clinical research.
Worn directly on the body, Libertas™ administers large-volume or highly viscous biotech drugs in doses between two and ten milliliters over a defined period of time. The application only requires a few steps. Furthermore, the needle is concealed before and after the injection process. Optionally, the injector can be networked with a Smart Device. That way, BD guarantees the safest and best possible therapy in the comfortable surroundings of your own home. A stable and perfectly performed joining process of the injector components is a basic requirement.
Suitable system for clinical trials
For the test volume production of the BD Libertas™, Harro Höfliger developed the semi-automatic Assembly Lab, a system for the assembly of patch injectors. During the earliest production phase, the system is able to provide process assurances for the final assembly process. The perfectly functioning ensemble consists of four parts: a cartridge containing active ingredient, an intermediate piece, a cover and the main assembly, which includes the needle. The components are placed manually into a workpiece carrier and joined together at seven stations. The scale-up of the final assembly can be easily done.
About Becton Dickinson
 BD is a leading supplier of innovative patient and user safety technologies with headquarters in Franklin Lakes, NJ, USA. The company, with 50,000 employees, partners with international organizations to address the most pressing healthcare challenges for people around the world.
BD is a leading supplier of innovative patient and user safety technologies with headquarters in Franklin Lakes, NJ, USA. The company, with 50,000 employees, partners with international organizations to address the most pressing healthcare challenges for people around the world.
Download this article as PDF file
Photos: Becton Dickinson, Helmar Lünig