Syringes, injection needles, booklets, cartons, cases – until recently, a significant amount of the packing process for this product by AstraZeneca was done by hand. Global increases in demand for the medication, however, made it necessary to automate the packaging process within the production line.
For Harro Höfliger, that meant finding solutions to insert the syringes and injection needles into the plastic tray, package them into folding cartons and shipping cases, and finally arrange them on a pallet allowing for serialization and aggregation to meet current and future market demands. It also included systems that kept a close eye on quality control.
Another requirement was that the machine processes needed to be adapted to the packaging material with its complex design as far as possible. This was achieved, among others, with a tailor-made solution for the intricate separation of the nested plastic trays and cover.
“A collaborative approach throughout the project made it clear that in Harro Höfliger we had chosen the right partner for the turnkey line.”Paul Bradley, Project Manager at AstraZeneca
Bausch+Ströbel, an Excellence United partner, was also involved, delivering the machines to assemble the pre-filled syringes with plungers, and to label them. Such close collaboration with the customer, Bausch+Ströbel and other technology suppliers resulted in a turnkey line that showcases the strengths of Harro Höfliger’s MKT horizontal cartoning machine and MCP modular case packer.
“Reliable processes and seamless quality control were critical in order to meet the demand for our medication,” says Paul Bradley, Project Manager at AstraZeneca. He admits that the task was an ambitious one: “A set of the medication can be supplied with either two pre-filled syringes with needles or with just a single syringe and needle. As a result, Harro Höfliger received specifications for two formats with no changeover.”
Click here to discover the entire production and packaging line

Smooth handling
All in all, the production and packaging line is made up of seven stations, with several in-line control systems. They ensure that the delicate syringes do not break, splinter or are scratched, that the sterile components are not damaged, and that the patient only receives complete, flawlessly packaged products of the highest quality.
The process begins with the unloading, infeed, assembly and labeling of the pre-filled syringes. “Our combined expertise, along with precisely coordinated technology and project management processes, allowed us to quickly find solutions to seamlessly integrate our systems,” says Martin Kern, Sales Group Manager at Bausch+Ströbel. The next step introduces the denested plastic trays into the process. Servo-driven transfers tilt the syringes at a predefined angle to be able to precisely place them into a one- or two-syringe tray.

The high-performance MCP (Modular Case Packer) from Harro Höfliger scores with its optimum accessibility, fast format changeover and reproducible setting options.
The supply of blister-packed injection needles with a safety system continues to be executed manually. Operators insert them into the product mounts in strips of five. The special form of the mounts ensures that once the needles are automatically separated, they will be placed in the correct orientation, lying flat in the tray. This placement is crucial in ensuring that the plastic cover can be positioned and mounted optimally in a later step.
Tactile quality control checks whether each product’s cover is positioned 100 percent correctly, ensuring only perfectly closed trays will be brought to the cartoning machine. The feeder is specially adapted to the folding carton with its characteristically inclined folding closure.
Reliable cartoning
No set is packaged without patient information or booklets: supplements corresponding to market-specific guidelines are placed on the tray as it is being transferred to the cartoning machine. In the MKT machine products are inserted horizontally into the folding carton, 30 packs per minute for both required formats.
The subsequent weight control determines whether each folding carton is correctly packed. If it detects a deviation, the package in question is automatically rejected. The application of tamper-evident labels or vignettes ensures the required protection against manipulation and meets specific market requirements.
A control unit also checks the correct positioning here, along with the completeness and legibility of the printed variable information, which includes batch number, manufacturing date, expiration date and any requirements for serialization and aggregation as per market demands.
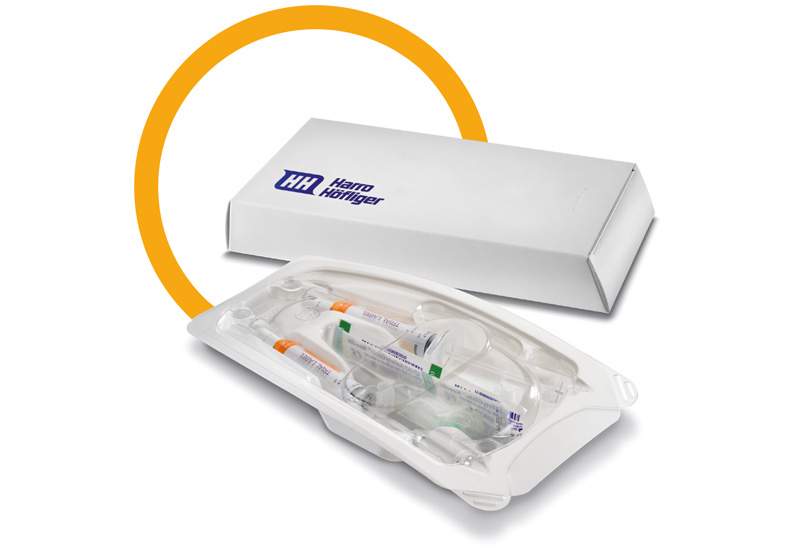
AstraZeneca’s successful oncology drug may consist of two ready-to-use glass syringes with a hypodermic needle or even just one syringe with a needle. For this reason, the specifications for Harro Höfliger provided for two formats without format changeover.
Efficiency with a new design
Harro Höfliger’s modular case packer MCP rounds out the end of the line. Apart from its efficiency in automatically stacking, packaging and sealing products, it also scores with the new turnkey design. The machine protection allows a complete overview of the packaging process, even better accessibility and an ergonomic work area.
15 folding cartons are fed on a single track, automatically grouped and stacked, then carefully side-loaded into the shipping case. The stacked cartons are aggregated with the help of a camera system. The case is then sealed with tape, labeled, affixed with a serial number and identified with a corner wrap label. After a camera system checks the code one last time, the cartons are assembled on a pallet.
About AstraZeneca
![]() AstraZeneca is a global, science-led, biopharmaceutical business, their innovative medicines are used by millions of patients worldwide.
AstraZeneca is a global, science-led, biopharmaceutical business, their innovative medicines are used by millions of patients worldwide.
Download this article as PDF file
Photos: Helmar Lünig, RAFF Digital, AstraZeneca
