The start-up enterprise PurelMS not only develops dry powder inhalers (DPIs) but also produces them in their own GMP compliant cleanroom. All products are based on the Cyclops™ platform and are also characterized by another common feature: “The ’pure‘ in our company name means that we use as few excipients as possible in our formulations,” says Floris Grasmeijer, Principal Scientist at PureIMS. “The special dispersing technology of our platform makes this possible without affecting drug efficacy. In most cases, we only need a small percentage of an excipient for our formulations, in some cases we require none at all. This is ideal for high-dose applications.”
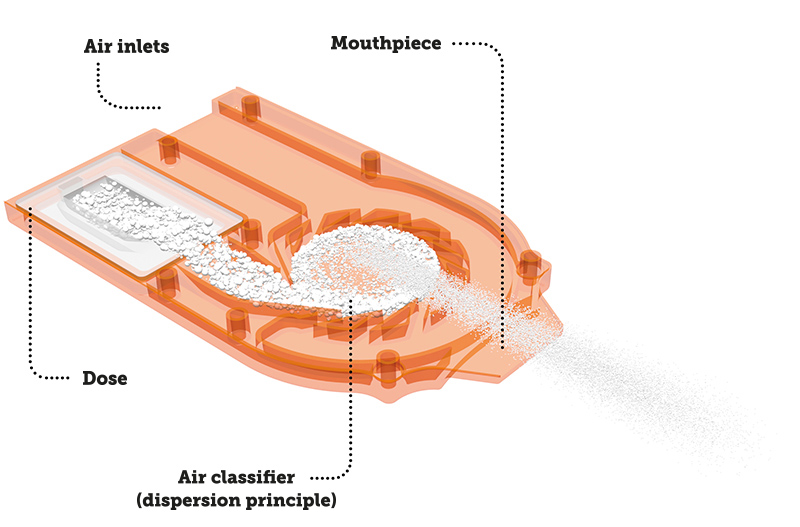
Bottom plate of the Cyclops™ during the inhalation process.
However, this specialization also poses particular challenges: “Commercially available formulations contain up to 95% lactose. This is what makes the powder flowable and enables reproducible dosing,” explains Grasmeijer. Pure formulations, on the other hand, have poor flow properties. Conventional filling techniques are therefore often not suitable for such powders.
Animated demonstration of the Cyclops™ dry powder inhaler:
Looking for options to scale and automate

The dry powder before (right) and after pressing into powder compacts (left).
“At the beginning, the entire powder filling process was done manually. The reproducible dosing of our cohesive formulations was not a topic at that time,” recalls Floris Grasmeijer. “Of course, the whole thing was very time consuming – and we rather wanted to spend our limited resources on development. So, we were looking for options to scale and automate the process. Already at that time we had good contacts with Harro Höfliger and thus came across the Omnidose dosing machine with drum filler technology.”
Numerous filling tests
The transition from manual dosing to the semi-automatic filling process was tricky: “The drum filler of the Omnidose forms the powder into small pellets and our inhaler platform was not yet adapted to this process,” explains Grasmeijer. “This is why we carried out numerous filling tests in Harro Höfliger‘s cleanrooms. With the support of the experts on site, we were able to adapt our inhalers in such a way that it became possible to process the pellets. Today we have our own Omnidose, which is a real eye-catcher in our cleanroom.”
“The drum filler of the Omnidose forms the powder into small pellets and our inhaler platform was not yet adapted to this process.”Floris Grasmeijer, Principal Scientist at PureIMS
Into the grown-up world
These extensive tests have paid off: “We now spend much less time on production and can focus more on development and the search for partners to finance the later development phases of our DPI products. The scalability of dosing processes and of course the well-established name Harro Höfliger have proven to be major advantages in discussions with potential partners.”

The PureIMS start-up team at the 2019 “Drug Delivery to the Lungs” exhibition.
PureIMS has big plans for the future: “We are still a small biopharmaceutical company. But things may change very quickly. We have the right products, are continuously expanding our process knowledge in the field of drug development and also have the appropriate production equipment. So we are making our way into the grown-up world – even if we will never behave like them,” says Floris Grasmeijer, tongue in cheek. “And when we get there, we will of course remember the push in the right direction which Harro Höfliger once gave us.”
About PureIMS

Pure Inhalation Medication Systems (PureIMS) is a biopharmaceutical company based in Roden, The Netherlands. The core activities of PureIMS are: development, manufacturing and commercialization of inhaled drugs for patients with diseases such as Cystic Fibrosis, Tuberculosis, Parkinson’s disease and Anaphylaxis. Cyclops™, a proprietary disposable dry powder inhaler, forms the innovative heart of all the therapeutic products.
Download this article as PDF file
Photos: PureIMS
