Sometimes every second counts – for example during medical emergencies. This is one of the major advantages of nasally administered drugs: The active ingredients reach the bloodstream via the nasal mucosa in a very short period of time with a fast onset of effect.
This is why Aptar Pharma’s Unit Dose System (UDS) is based on the nasal administration of powder. The sophisticated design of the one-handed disposable device enables the reliable, systemic delivery of active ingredients in precise doses; even when administered by the patients themselves.
No matter which active ingredient is administered with the device, Harro Höfliger offers equipment for all phases of development, from purely manual to semi-automatic production, all the way to fully automatic turnkey solutions. Clinical samples can be produced directly at the strategic partner Experic. This cooperation between Aptar Pharma, Experic and Harro Höfliger gives pharmaceutical customers the opportunity to develop new drugs reliably and quickly without having to invest in their own equipment in early phases.
They benefit from know-how and comprehensive services as early as the preclinical and clinical development stage. In addition, scalable processes across all development stages ensure resource-saving development and maximum process reliability.
 An important component of the UDS device is the container. This cartridge contains a so-called “mandrel” made of plastic, a ball and the active ingredient. When the patient activates the inhaler, the mandrel pushes the ball upwards and the active ingredient is released into the nose.
An important component of the UDS device is the container. This cartridge contains a so-called “mandrel” made of plastic, a ball and the active ingredient. When the patient activates the inhaler, the mandrel pushes the ball upwards and the active ingredient is released into the nose.
Filling and assembly in 3 development stages:
1. Manual production

If pharmaceutical customers want to use Aptar Pharma’s device for their formulation, early filling tests can be performed directly in Harro Höfliger’s Pharma Services department. This allows easy and quick insights into the interaction between device, powder formulation and process.
Many questions need to be clarified during this proof-of-concept phase: What quantity of powder can be dosed? Can the powder be easily processed? Is the device capable of dispensing the desired amount of powder? In order to examine these points, the experts use the purely manual dosing machine Drum TT. In addition, there is equipment for the simple, reliable and reproducible final assembly of the container, for example hand presses for pressing the ball precisely into the container, and for the standardized assembly of all other device components.
2. Semi-automatic solution

The filling process developed in the laboratory is the basis for the next step, which involves a Drum Lab for the semi-automatic filling of the device. The components are inserted manually and the container is filled fully automatically.
With an output of approximately five to ten UDS devices per minute, the equipment is ideally suited for the early phases of clinical studies. An integrated weighing system and the batch report for seamless documentation of the filling process ensure that all requirements of pharmaceutical development are met.
Therefore the Drum Lab – together with the hand presses and the process expertise gained during manual production – can be transferred to Experic for the production of clinical test samples. Experic not only provides support in terms of production, but with its extensive know-how also in all other challenges related to clinical studies, for example in logistics.
3. Fully automatic production
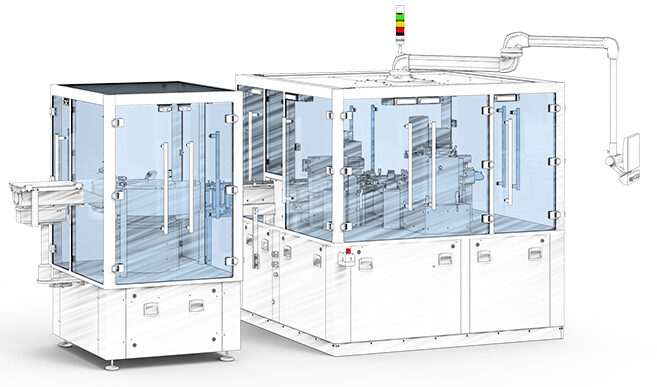
At present, one system for the fully automatic filling of UDS containers is already in operation at a customer’s site; another one is at the design stage. The dosing process can be easily scaled on the basis of the previous steps. Also pressing-in of the ball as well as the assembly of the components is based on the experience gained from previous development phases. In addition, integrated control systems ensure high product quality. With an output of around 50 devices per minute, the fully automated systems are suited for both larger clinical studies and commercial production. If desired, both applications can be placed directly with Experic.
About Aptar Pharma
 Aptar Pharma is part of AptarGroup, Inc., a global leader in the design and manufacturing of a broad range of drug delivery, consumer product dispensing and active packaging solutions. The company is headquartered in Crystal Lake, Illinois and has 14,000 dedicated employees in 20 countries.
Aptar Pharma is part of AptarGroup, Inc., a global leader in the design and manufacturing of a broad range of drug delivery, consumer product dispensing and active packaging solutions. The company is headquartered in Crystal Lake, Illinois and has 14,000 dedicated employees in 20 countries.
About Experic
![]() The US-based company Experic with headquarters in Cranbury, New Jersey, supports pharmaceutical companies in all aspects of clinical trials, for example in the production of test samples. Experic’s extensive technology portfolio includes all the equipment required for the filling and assembly of the UDS device.
The US-based company Experic with headquarters in Cranbury, New Jersey, supports pharmaceutical companies in all aspects of clinical trials, for example in the production of test samples. Experic’s extensive technology portfolio includes all the equipment required for the filling and assembly of the UDS device.
Download this article as PDF file
Photos: Adobe Stock/ac2000, Aptar Pharma
